Continuous Manufacturing: A Shift from Batch Processing
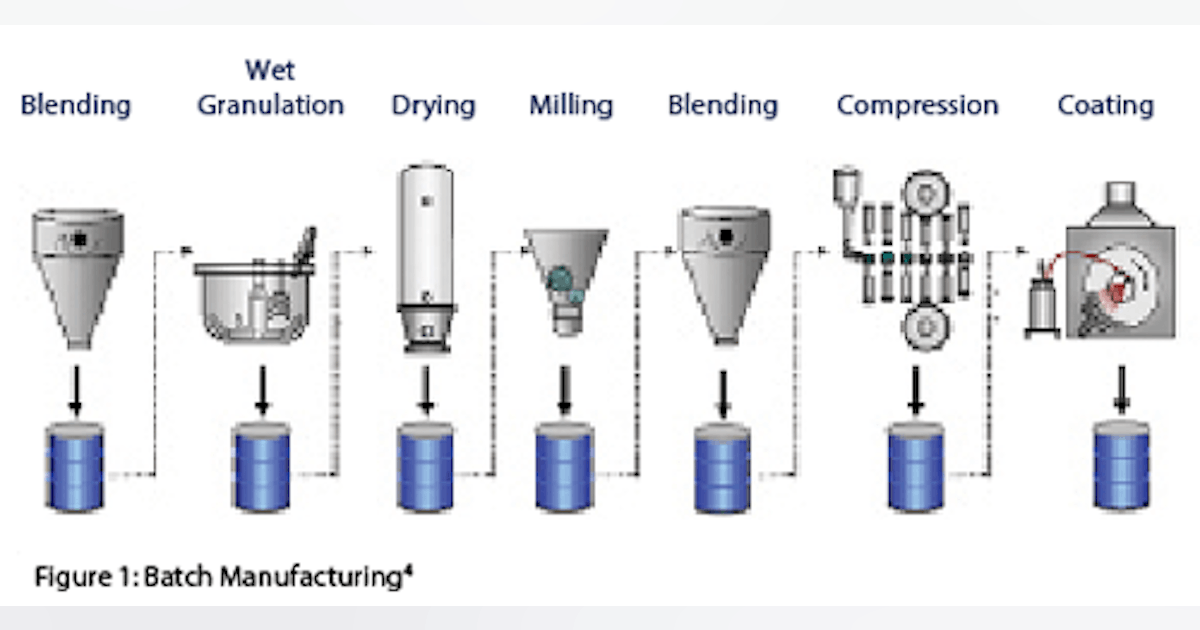
1. Introduction The pharmaceutical industry has traditionally relied on batch processing, where drugs are manufactured in discrete steps before moving to the next stage. While effective, this approach can be time-consuming, resource-intensive, and prone to inefficiencies. A major shift is happening towards continuous manufacturing (CM), a process where drug production occurs in a seamless, uninterrupted flow. This transformation enhances efficiency, scalability, and quality control, enabling pharma companies to produce medicines faster and at a lower cost. Regulatory agencies like the FDA and EMA are actively promoting continuous manufacturing as a way to improve drug availability, reduce shortages, and enhance safety. This article explores continuous manufacturing in pharma, its benefits over batch processing, and how it’s shaping the future of drug production.
2. What is Continuous Manufacturing in Pharma?
Continuous Manufacturing (CM) is a modern drug production method where raw materials are continuously fed into the system, and finished pharmaceutical products are consistently produced without interruptions.
Unlike batch processing, where production is paused between steps, continuous manufacturing operates as a fully automated system, allowing for:
- Real-time monitoring and adjustments
- Seamless integration of production stages
- Higher precision and efficiency
Continuous manufacturing is transforming the pharmaceutical landscape by reducing manual intervention and ensuring consistent drug quality.